Help us make food transparency the norm!
As a non-profit organization, we depend on your donations to continue informing consumers around the world about what they eat.
The food revolution starts with you!
Nextar Soft Brownies - Nabati - 36 g
Nextar Soft Brownies - Nabati - 36 g
Barcode: 8993175549387 (EAN / EAN-13)
Common name: Cookies with cocoa filling
Quantity: 36 g
Brands: Nabati
Categories: Snacks, Sweet snacks, Biscuits and cakes, Biscuits, Chocolate biscuits
Labels, certifications, awards:
Vegetarian, Green Dot India
Origin of the product and/or its ingredients: Indonesia
Origin of ingredients: Indonesia
Countries where sold: India
Matching with your preferences
Health
Ingredients
-
40 ingredients
Cookies (77.8%) (Refined Wheat Flour (Maida), Glucose Syrup, Edible Vegetable Fat ((Hydrogenated Palm Olein) (contains Antioxidant (INS 319))), Sugar, Cocoa Powder (4.50% ), Edible Vegetable Oil ((Palm Olein) (contains Antioxidant (INS 319))), Humectant (INS 422), Palm Sugar, Emulsifier (INS 322(i)), Leavening Agent (INS 500(ii)), Edible Common Salt, Artificial Vanilla Flavour, Preservative (INS 202), Antioxidant (INS 307a), Acidity Regulator (INS 330)). Cocoa Cream Filling (22.2%) (Sugar, Edible Vegetable Fat ((Hydrogenated Palm Olein) (contains Antioxidant (INS 319))), Cocoa Powder (1.89%), Maltodextrin, Emulsifier (INS 322(i)), Edible Common Salt).Allergens: Gluten, Soybeans
Food processing
-
Ultra processed foods
Elements that indicate the product is in the 4 - Ultra processed food and drink products group:
- Additive: E322 - Lecithins
- Additive: E422 - Glycerol
- Ingredient: Emulsifier
- Ingredient: Glucose
- Ingredient: Glucose syrup
- Ingredient: Humectant
- Ingredient: Maltodextrin
Food products are classified into 4 groups according to their degree of processing:
- Unprocessed or minimally processed foods
- Processed culinary ingredients
- Processed foods
- Ultra processed foods
The determination of the group is based on the category of the product and on the ingredients it contains.
Additives
-
E202 - Potassium sorbate
Potassium sorbate (E202) is a synthetic food preservative commonly used to extend the shelf life of various food products.
It works by inhibiting the growth of molds, yeast, and some bacteria, preventing spoilage. When added to foods, it helps maintain their freshness and quality.
Some studies have shown that when combined with nitrites, potassium sorbate have genotoxic activity in vitro. However, potassium sorbate is generally recognized as safe (GRAS) by regulatory authorities.
-
E307a - D-Alpha-tocopherol
Alpha-Tocopherol: α-Tocopherol is a type of vitamin E. It has E number "E307". Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain which allows for penetration into biological membranes. Compared to the others, α-tocopherol is preferentially absorbed and accumulated in humans.Source: Wikipedia
-
E319 - Tertiary-butylhydroquinone (tbhq)
Tert-Butylhydroquinone: tert-Butylhydroquinone -TBHQ, tertiary butylhydroquinone- is a synthetic aromatic organic compound which is a type of phenol. It is a derivative of hydroquinone, substituted with a tert-butyl group.Source: Wikipedia
-
E322 - Lecithins
Lecithins are natural compounds commonly used in the food industry as emulsifiers and stabilizers.
Extracted from sources like soybeans and eggs, lecithins consist of phospholipids that enhance the mixing of oil and water, ensuring smooth textures in various products like chocolates, dressings, and baked goods.
They do not present any known health risks.
-
E322i - Lecithin
Lecithins are natural compounds commonly used in the food industry as emulsifiers and stabilizers.
Extracted from sources like soybeans and eggs, lecithins consist of phospholipids that enhance the mixing of oil and water, ensuring smooth textures in various products like chocolates, dressings, and baked goods.
They do not present any known health risks.
-
E330 - Citric acid
Citric acid is a natural organic acid found in citrus fruits such as lemons, oranges, and limes.
It is widely used in the food industry as a flavor enhancer, acidulant, and preservative due to its tart and refreshing taste.
Citric acid is safe for consumption when used in moderation and is considered a generally recognized as safe (GRAS) food additive by regulatory agencies worldwide.
-
E422 - Glycerol
Glycerol: Glycerol -; also called glycerine or glycerin; see spelling differences- is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in all lipids known as triglycerides. It is widely used in the food industry as a sweetener and humectant and in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature.Source: Wikipedia
-
E500 - Sodium carbonates
Sodium carbonates (E500) are compounds commonly used in food preparation as leavening agents, helping baked goods rise by releasing carbon dioxide when they interact with acids.
Often found in baking soda, they regulate the pH of food, preventing it from becoming too acidic or too alkaline. In the culinary world, sodium carbonates can also enhance the texture and structure of foods, such as noodles, by modifying the gluten network.
Generally recognized as safe, sodium carbonates are non-toxic when consumed in typical amounts found in food.
-
E500ii - Sodium hydrogen carbonate
Sodium hydrogen carbonate, also known as E500ii, is a food additive commonly used as a leavening agent.
When added to recipes, it releases carbon dioxide gas upon exposure to heat or acids, causing dough to rise and resulting in a light, fluffy texture in baked goods.
It is generally recognized as safe (GRAS) by regulatory authorities when used in appropriate quantities and poses no significant health risks when consumed in typical food applications.
Ingredients analysis
-
Palm oil
Ingredients that contain palm oil: Palm olein, Palm olein, Palm olein
-
Vegan status unknown
Unrecognized ingredients: Cookies, Artificial-vanilla-flavour, Cocoa-cream-fillingSome ingredients could not be recognized.
We need your help!
You can help us recognize more ingredients and better analyze the list of ingredients for this product and others:
- Edit this product page to correct spelling mistakes in the ingredients list, and/or to remove ingredients in other languages and sentences that are not related to the ingredients.
- Add new entries, synonyms or translations to our multilingual lists of ingredients, ingredient processing methods, and labels.
If you would like to help, join the #ingredients channel on our Slack discussion space and/or learn about ingredients analysis on our wiki. Thank you!
-
Vegetarian
No non-vegetarian ingredients detected
Unrecognized ingredients: Cookies, Artificial-vanilla-flavour, Cocoa-cream-fillingSome ingredients could not be recognized.
We need your help!
You can help us recognize more ingredients and better analyze the list of ingredients for this product and others:
- Edit this product page to correct spelling mistakes in the ingredients list, and/or to remove ingredients in other languages and sentences that are not related to the ingredients.
- Add new entries, synonyms or translations to our multilingual lists of ingredients, ingredient processing methods, and labels.
If you would like to help, join the #ingredients channel on our Slack discussion space and/or learn about ingredients analysis on our wiki. Thank you!
-
Details of the analysis of the ingredients
We need your help!
Some ingredients could not be recognized.
We need your help!
You can help us recognize more ingredients and better analyze the list of ingredients for this product and others:
- Edit this product page to correct spelling mistakes in the ingredients list, and/or to remove ingredients in other languages and sentences that are not related to the ingredients.
- Add new entries, synonyms or translations to our multilingual lists of ingredients, ingredient processing methods, and labels.
If you would like to help, join the #ingredients channel on our Slack discussion space and/or learn about ingredients analysis on our wiki. Thank you!
: Cookies 77.8%, Refined Wheat Flour (Maida), Glucose Syrup, Edible Vegetable Fat (Palm Olein, contains Antioxidant (e319)), Sugar, Cocoa Powder 4.5%, Edible Vegetable Oil (Palm Olein, contains Antioxidant (e319)), Humectant (e422), Palm Sugar, Emulsifier (e322i), Leavening Agent (e500ii), Edible Common Salt, Artificial Vanilla Flavour, Preservative (e202), Antioxidant (e307a), Acidity Regulator (e330), Cocoa Cream Filling 22.2%, Sugar, Edible Vegetable Fat (Palm Olein, contains Antioxidant (e319)), Cocoa Powder 1.89%, Maltodextrin, Emulsifier (e322i), Edible Common Salt- Cookies -> en:cookies - percent: 77.8
- Refined Wheat Flour -> en:refined-wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410
- Maida -> en:refined-wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410
- Glucose Syrup -> en:glucose-syrup - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016
- Edible Vegetable Fat -> en:vegetable-fat - vegan: yes - vegetarian: yes - from_palm_oil: maybe
- Palm Olein -> en:palm-olein - vegan: yes - vegetarian: yes - from_palm_oil: yes - ciqual_food_code: 16129
- contains Antioxidant -> en:antioxidant
- e319 -> en:e319 - vegan: yes - vegetarian: yes
- Sugar -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016
- Cocoa Powder -> en:cocoa-powder - vegan: yes - vegetarian: yes - ciqual_food_code: 18100 - percent: 4.5
- Edible Vegetable Oil -> en:vegetable-oil - vegan: yes - vegetarian: yes - from_palm_oil: maybe
- Palm Olein -> en:palm-olein - vegan: yes - vegetarian: yes - from_palm_oil: yes - ciqual_food_code: 16129
- contains Antioxidant -> en:antioxidant
- e319 -> en:e319 - vegan: yes - vegetarian: yes
- Humectant -> en:humectant
- e422 -> en:e422 - vegan: maybe - vegetarian: maybe
- Palm Sugar -> en:palm-sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016
- Emulsifier -> en:emulsifier
- e322i -> en:e322i - vegan: maybe - vegetarian: maybe
- Leavening Agent -> en:raising-agent
- e500ii -> en:e500ii - vegan: yes - vegetarian: yes
- Edible Common Salt -> en:salt - vegan: yes - vegetarian: yes - ciqual_food_code: 11058
- Artificial Vanilla Flavour -> en:artificial-vanilla-flavour
- Preservative -> en:preservative
- e202 -> en:e202 - vegan: yes - vegetarian: yes
- Antioxidant -> en:antioxidant
- e307a -> en:e307a - vegan: yes - vegetarian: yes
- Acidity Regulator -> en:acidity-regulator
- e330 -> en:e330 - vegan: yes - vegetarian: yes
- Cocoa Cream Filling -> en:cocoa-cream-filling - percent: 22.2
- Sugar -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016
- Edible Vegetable Fat -> en:vegetable-fat - vegan: yes - vegetarian: yes - from_palm_oil: maybe
- Palm Olein -> en:palm-olein - vegan: yes - vegetarian: yes - from_palm_oil: yes - ciqual_food_code: 16129
- contains Antioxidant -> en:antioxidant
- e319 -> en:e319 - vegan: yes - vegetarian: yes
- Cocoa Powder -> en:cocoa-powder - vegan: yes - vegetarian: yes - ciqual_food_code: 18100 - percent: 1.89
- Maltodextrin -> en:maltodextrin - vegan: yes - vegetarian: yes
- Emulsifier -> en:emulsifier
- e322i -> en:e322i - vegan: maybe - vegetarian: maybe
- Edible Common Salt -> en:salt - vegan: yes - vegetarian: yes - ciqual_food_code: 11058
Nutrition
-
Bad nutritional quality
⚠ ️Warning: the amount of fruits, vegetables and nuts is not specified on the label, it was estimated from the list of ingredients: 0This product is not considered a beverage for the calculation of the Nutri-Score.
Positive points: 0
- Proteins: 3 / 5 (value: 5, rounded value: 5)
- Fiber: 0 / 5 (value: 0, rounded value: 0)
- Fruits, vegetables, nuts, and colza/walnut/olive oils: 0 / 5 (value: 0, rounded value: 0)
Negative points: 20
- Energy: 5 / 10 (value: 1971, rounded value: 1971)
- Sugars: 5 / 10 (value: 25, rounded value: 25)
- Saturated fat: 8 / 10 (value: 9, rounded value: 9)
- Sodium: 2 / 10 (value: 242, rounded value: 242)
The points for proteins are not counted because the negative points are greater or equal to 11.
Nutritional score: (20 - 0)
Nutri-Score:
-
Nutrient levels
-
Fat in moderate quantity (20%)
What you need to know- A high consumption of fat, especially saturated fats, can raise cholesterol, which increases the risk of heart diseases.
Recommendation: Limit the consumption of fat and saturated fat- Choose products with lower fat and saturated fat content.
-
Saturated fat in high quantity (9%)
What you need to know- A high consumption of fat, especially saturated fats, can raise cholesterol, which increases the risk of heart diseases.
Recommendation: Limit the consumption of fat and saturated fat- Choose products with lower fat and saturated fat content.
-
Sugars in high quantity (25%)
What you need to know- A high consumption of sugar can cause weight gain and tooth decay. It also augments the risk of type 2 diabetes and cardio-vascular diseases.
Recommendation: Limit the consumption of sugar and sugary drinks- Sugary drinks (such as sodas, fruit beverages, and fruit juices and nectars) should be limited as much as possible (no more than 1 glass a day).
- Choose products with lower sugar content and reduce the consumption of products with added sugars.
-
Salt in moderate quantity (0.605%)
What you need to know- A high consumption of salt (or sodium) can cause raised blood pressure, which can increase the risk of heart disease and stroke.
- Many people who have high blood pressure do not know it, as there are often no symptoms.
- Most people consume too much salt (on average 9 to 12 grams per day), around twice the recommended maximum level of intake.
Recommendation: Limit the consumption of salt and salted food- Reduce the quantity of salt used when cooking, and don't salt again at the table.
- Limit the consumption of salty snacks and choose products with lower salt content.
-
-
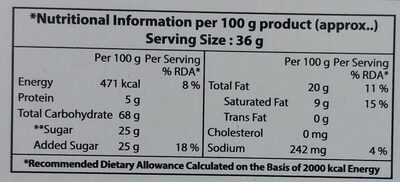
Nutrition facts
Nutrition facts As sold
for 100 g / 100 mlAs sold
per serving (36 g)Compared to: Chocolate biscuits Energy 1,971 kj
(471 kcal)710 kj
(170 kcal)-3% Fat 20 g 7.2 g -3% Saturated fat 9 g 3.24 g -2% Carbohydrates 68 g 24.5 g -2% Sugars 25 g 9 g -26% Fiber 0 g 0 g Proteins 5 g 1.8 g +4% Salt 0.605 g 0.218 g +127% Fruits‚ vegetables‚ nuts and rapeseed‚ walnut and olive oils (estimate from ingredients list analysis) 0 % 0 %
Environment
-
Eco-Score D - High environmental impact
⚠ ️The full impact of transportation to your country is currently unknown.The Eco-Score is an experimental score that summarizes the environmental impacts of food products.→ The Eco-Score was initially developped for France and it is being extended to other European countries. The Eco-Score formula is subject to change as it is regularly improved to make it more precise and better suited to each country.Life cycle analysis
-
Average impact of products of the same category: C (Score: 56/100)
Category: Biscuit (cookie), with chocolate, prepacked
Category: Biscuit (cookie), with chocolate, prepacked
- PEF environmental score: 0.47 (the lower the score, the lower the impact)
- including impact on climate change: 5.92 kg CO2 eq/kg of product
Stage Impact Agriculture
63.6 %Processing
29.8 %Packaging
2.3 %Transportation
3.2 %Distribution
1.0 %Consumption
0.0 %
Bonuses and maluses
-
Origins of ingredients with a high impact
Malus:
Environmental policy: -5
Transportation: 0
Origin of the product and/or its ingredients % of ingredients Impact Indonesia 100 %High
-
Ingredients that threatens species
Malus: -10
Contains palm oil
Tropical forests in Asia, Africa and Latin America are destroyed to create and expand oil palm tree plantations. The deforestation contributes to climate change, and it endangers species such as the orangutan, the pigmy elephant and the Sumatran rhino.
-
Packaging with a medium impact
Malus: -11
Shape Material Recycling Impact Unknown Cardboard Low Unknown Plastic High ⚠ ️ The information about the packaging of this product is not sufficiently precise (exact shapes and materials of all components of the packaging).⚠ ️ For a more precise calculation of the Eco-Score, you can modify the product page and add them.
If you are the manufacturer of this product, you can send us the information with our free platform for producers.
Eco-Score for this product
-
Impact for this product: D (Score: 30/100)
Product: Nextar Soft Brownies - Nabati - 36 g
Life cycle analysis score: 56
Sum of bonuses and maluses: -21
Final score: 30/100
-
Carbon footprint
-
Equal to driving 3.1 km in a petrol car
592 g CO² per 100g of product
The carbon emission figure comes from ADEME's Agribalyse database, for the category: Biscuit (cookie), with chocolate, prepacked (Source: ADEME Agribalyse Database)
Stage Impact Agriculture
52.9 %Processing
42.0 %Packaging
1.9 %Transportation
2.9 %Distribution
0.3 %Consumption
0.0 %
Packaging
-
Packaging with a medium impact
-
Packaging parts
(Cardboard)
(Plastic)
-
Packaging materials
Material % Packaging weight Packaging weight per 100 g of product Paper or cardboard Plastic Total
-
Transportation
-
Origins of ingredients
Origins of ingredients with a high impact
Origin of the product and/or its ingredients % of ingredients Impact Indonesia 100 %High
Threatened species
-
Contains palm oil
Drives deforestation and threatens species such as the orangutan
Tropical forests in Asia, Africa and Latin America are destroyed to create and expand oil palm tree plantations. The deforestation contributes to climate change, and it endangers species such as the orangutan, the pigmy elephant and the Sumatran rhino.
Report a problem
-
Incomplete or incorrect information?
Category, labels, ingredients, allergens, nutritional information, photos etc.
If the information does not match the information on the packaging, please complete or correct it. Open Food Facts is a collaborative database, and every contribution is useful for all.
Data sources
Product added on by chunkieramos
Last edit of product page on by chunkieramos.
Product page also edited by roboto-app.